To reach the equivalence point in

The equivalence point of the titration is the point at which exactly enough

At the third equivalence point, only the weak base PO43- is in the solution.

titration curve with sharp jump from pH = 2 to pH = 11 as equivalence point

Moreover, the equivalence point itself is shifted upward in pH.

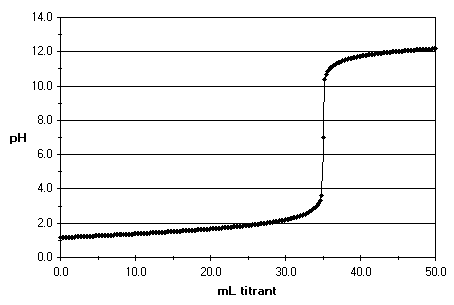
The slope of the titration curve is steepest at the equivalence point,

How to Find an Equivalence Point Titration

In the weak acid titration, thymol blue changes colour at the equivalence

Near the equivalence point there is a rapid increase in pH.

At the stoichiometric or equivalence point (S on the curve),

At the equivalence point the solution contains only

we have reached the equivalence point in such a titration experiment?
The real equivalence point would be between 22.5 and 25.0 -- and probably
point; equivalence point; right of equivalence point. Titration of a

Chemistry: Calculating pH at various points on a titration curve.
To clear confusion, the endpoint and equivalence point are not necessarily

This is not the only method to determine the equivalence point,
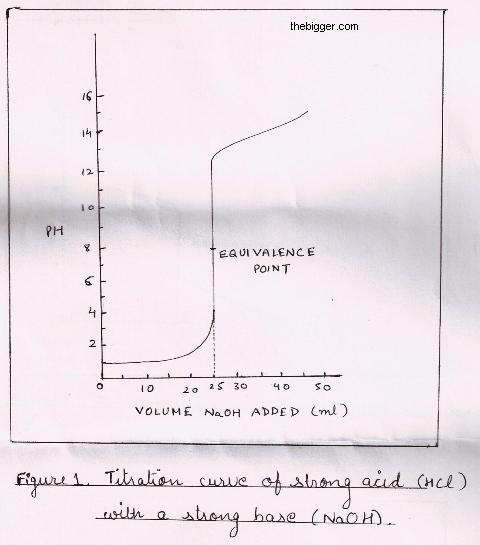
It is observed that at the equivalence point pH is 7 but the pH sharply

to the equivalence point than in a strong acid-strong base titration,

The pH decreases slowly in the beginning and near the equivalence point

0 comments:
Post a Comment